Hyperkalemia
definition
A Hyperkalemia occurs when the level of potassium in the blood exceeds a certain level. Exceeds the potassium concentration in the blood serum 5 mmol / l, one speaks of an excess in adults. The limit value in childhood is 5.4 mmol / l.
Usually most of the potassium is inside the cell. Only about two percent circulate in the so-called extracellular space. The difference in concentration serves to maintain the membrane potential between the inside and outside of the cells. Even small changes in concentration have significant effects on the Cardiovascular system and the interaction of Muscles and nerves. Such an electrolyte disturbance can life threatening be. However, it is not just the fluctuation in concentration that plays an important role, but also the speed with which it occurs. The faster the serum potassium rises, the more serious the consequences.

There are several causes of a potassium increase Kidney disease, how acute kidney failure, Addison's disease and chronic renal failure. Potassium, which is normally excreted, stays in the body.
Also Medication can cause hyperkalemia. In addition, lead Changes in pH, extensive destruction of muscles or increased potassium intake resulting in an excess of electrolytes. Incorrectly high values when taking blood samples are due to the leakage of potassium from burst red blood cells.
These symptoms indicate hyperkalemia
Hyperkalemia is an emergency because, in the worst case scenario, it can lead to severe cardiac arrhythmia and cardiac arrest. The following symptoms are typical of the disease and should be accurately diagnosed.
Cardiac arrhythmias
There are many causes that can cause the heart to lose its rhythm. One cause is hyperkalemia. The too high proportion of potassium leads to permanent excitation on the cell walls of the heart muscle cells. The permanent excitation then ensures that the cells go to rest in a disordered manner and are no longer controlled regularly.
This causes irregular heartbeats and an irregular flow of blood through the body. This can lead to what is known as ventricular fibrillation, which is tantamount to cardiac arrest, as the heart beats too quickly to pump blood around the body.
Also read: Cardiac arrhythmia
Acidosis
Acidosis is excess acidity in the blood. The pH value, which indicates how acidic the blood is, is in a narrow range and deviations can have serious consequences.
In acidosis, the body tries to balance the value through the kidneys. This means that acidic protons are excreted. However, the kidneys can only do this in direct exchange for potassium ions. For every proton that is released, the body absorbs one potassium ion. The amount of potassium in the blood increases and hyperkalaemia occurs with the corresponding consequences.
Read more here: Acidosis
A slow heartbeat
Bradycardia, i.e. a slow heartbeat, can have many causes. Bradycardia is an abnormal heart rhythm.
An excess of potassium in the heart muscle cells can lead to certain ion channels being disordered during a kind of rest. As a result, the heartbeats are no longer triggered regularly. This can lead to various cardiac arrhythmias, one of which is bradycardia.
You may also be interested in the following article: Bradycardia
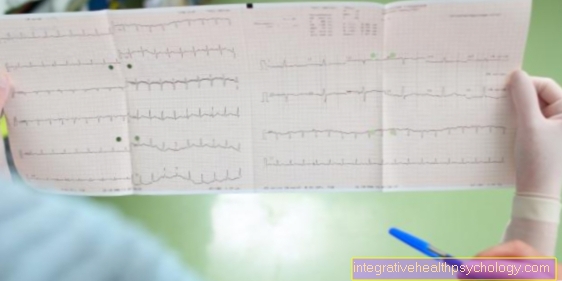
EKG changes
In the electrocardiogram, or ECG for short, the doctor can identify certain heart diseases. The patterns in the EKG are typically shaped in certain disorders, including hyperkalaemia.
The first thing you notice are the irregular heartbeats and the so-called tent-shaped T. This is the final wave of a complete heart rhythm complex. If there is a strong excess of potassium, the other waves in the ECG also change.
With ventricular fibrillation, which can be a consequence of hyperkalemia, the EKG shows very rapid, disordered peaks. An EKG can be recorded both routinely by the family doctor and for permanent control in the hospital.
Read more on this topic at: electrocardiogram
Metallic taste in the mouth
A metallic taste in the mouth can be caused by various diseases and drugs. With kidney failure, those affected often report a metallic taste.
Kidney failure is one of the most common causes of hyperkalaemia and therefore patients with hyperkalaemia often report the change in taste. However, the metallic taste is not triggered directly by potassium in the mouth. The person concerned does not taste potassium, but the sensory perception of taste changes overall.
Read more in the following article: Symptoms of kidney failure
Pain
Hyperkalemia shows very unspecific symptoms and is usually not associated with pain. However, some people affected may find the tingling in their fingers, which is typical for hyperkalemia, very unpleasant and therefore report pain.
In the case of acute kidney failure due to inflammation, which can be a cause of hyperkalemia, severe pain in the kidney area can also occur. However, these are not the result of hyperkalemia.
Read more on the subject at: Acute kidney failure
fatigue
Since hyperkalemia always occurs in the context of other diseases, it can be difficult to assign a symptom such as tiredness to a specific cause.
In most cases, however, the cause is not hyperkalemia, but an additional symptom in addition to fatigue. Fatigue is much more typical with hypokalaemia, i.e. with a drop in the potassium level.
Read more on the subject at: Hypokalemia
constipation
Constipation is also more of a symptom of hypokalemia. This means that the person has too little potassium in their blood. Constipation is uncommon for hyperkalemia.
However, since many symptoms are due to the underlying disease and not to the hyperkalemia, constipation can coincide with the hyperkalemia. This is possible, for example, with tumor diseases in the intestine. With some therapies, the tumor can be attacked so quickly that the tumor dissolves and the components lead to disturbances in the body's salt and water balance.
Read more on the subject at: Colon cancer
Muscle weakness
One of the two typical symptoms of hyperkalemia is muscle weakness. This affects both the skeletal muscles and the heart muscles. The increased potassium opens up ion channels in the cell membranes. After each opening, the channels take a short break. Due to the increased amount of potassium, this cycle gets out of rhythm and the cells receive different information. In the case of the skeletal muscles, this means that those affected can muster less strength.
Also read: Muscle weakness
Clouding of consciousness
Hyperkalemia is an absolute emergency and can have serious consequences for the body within a short period of time. After the rather unspecific early symptoms, there is a cardiac arrhythmia with a heart beating too slowly. This slow heartbeat can no longer supply the body with sufficient oxygen-rich blood.
The brain needs large amounts of oxygen and even a small deficiency can trigger a disturbance of consciousness. This is a protective mechanism of the body, as the brain uses less oxygen in a sleep mode.
Emergency Medicine Guidelines
In the emergency medical There are guidelines for the adequate diagnosis and treatment of electrolyte disorders caused by hyperkalaemia. There is no separate guideline for hyperkalemia. However, it is mentioned in other guidelines, for example in arterial hypertension.
In clinical diagnostics, the determination of electrolytes, a blood gas analysis, kidney values and the ECG play an important role.
Come as therapeutic agents Diuretics, Infusions from Glucose and insulin, the compensation of the acidic pH value by means of infusion and the treatment of changes in the EKG are used. So-called cation exchangers, for example Resonium, bind potassium in exchange for sodium in the intestine.
Three to four hour hemodialysis is used to eliminate potassium from outside the body and is considered for particularly high potassium levels.
Therapy with glucose and insulin
If hyperkalemia becomes symptomatic, it is acutely life-threatening condition. Therapy must take place immediately. Various measures are taken to lower the potassium concentration. One of them is the gift of insulin. The application takes place either as an injection or as a continuous infusion. The infusion contains a precisely calculated amount of insulin and glucose.
insulin causes glucose to be absorbed into the cells of the liver and skeletal muscles. At the same time, potassium is also transported into the cells and thus removed from the extracellular space. The administration of insulin alone would lead to hypoglycaemia if the blood sugar level is normal. For this reason, glucose is added to the infusion. However, this has no effect on the level of the potassium value. In general, the blood sugar must be checked at short intervals during the administration of insulin.
Insulin can be administered as a so-called bolus in the form of 10 to 20 IU (injection units) into the subcutaneous fatty tissue. Another option is intravenous administration via a continuous infusion. For example, 10 IU insulin is administered together with 100 ml of a 33 percent glucose solution. However, the exact dosage depends on the starting blood sugar level. The first effects set in after about 10 to 20 minutes. The maximum effect is reached after about half an hour to a full hour and lasts in decreasing strength for about five hours. The potassium level can be reduced by a value of 0.5 to 1.5 mmol / l during this time.
The higher the value of the original potassium concentration and the higher the added insulin concentration, the more pronounced the therapeutic effect.
The infusion of insulin represents a efficient and fast method to lower the serum potassium concentration. Only the dialysis achieves an even faster decrease.
ACE inhibitors
ACE inhibitors are mainly used in the therapy of arterial hypertension, i.e. increased blood pressure. One consequence is the inhibition of the renin-angiotensin-aldosterone system (RAAS), which means that less aldosterone is released. In less than 10% of cases, this causes an increase in potassium in the serum, i.e. hyperkalaemia. This side effect does not occur in small doses.
The following risk factors also increase the likelihood of a potassium excess: already present Renal failure, Heart failure and high age. The parallel intake non-steroidal anti-inflammatory drugs (NSAIDs) and potassium-sparing diuretics also promote the occurrence of hyperkalemia.
Alkalosis
Changes in pH affect the potassium concentration.
A falling, i.e. acidic, pH value (= Acidosis), causes a redistribution of the ions. The concentration of potassium in the serum increases.
In the therapy of hyperkalemia, the opposite effect is used to lower the potassium value.
The administration of sodium hydrogen carbonate increases the pH value. This creates an alkalosis, which lowers the potassium concentration in the serium. A side effect of therapy with sodium hydrogen carbonate is consequently the Alkalosis by increasing the pH.










.jpg)